Data Management

Recruiting participants for clinical trials is challenging, but social media is changing the game. Platforms like Facebook and Instagram enable researchers to target specific demographics, reaching a broader and more diverse audience quickly and cost-effectively. This blog explores how social media enhances recruitment efforts, the strategies for effective use, and the potential challenges involved.

Explore how digital health platforms are revolutionizing clinical research. Discover the impact of technologies like EHRs, mobile apps, and wearables on patient recruitment, data collection, and trial efficiency. Learn how these tools overcome traditional challenges and accelerate the development of new therapies. Dive into the future of clinical trials with digital innovation.

Discover how Real-World Evidence (RWE) is transforming regulatory practices. RWE, derived from sources like health records and insurance claims, offers a broader view of treatment efficacy beyond controlled trials. This blog highlights RWE's role in accelerating approvals, enhancing personalized care, and addressing challenges in data quality and privacy. Explore how regulatory bodies are adapting to this valuable data for more informed decision-making.

Precision medicine is revolutionizing clinical trials by personalizing treatments through genetic and lifestyle insights. This blog explores how this approach enhances efficacy, reduces costs, and introduces adaptive trial designs, while addressing challenges like high costs and data privacy. Discover the future of tailored healthcare and its implications for clinical research.
Discover how data analytics is transforming clinical research by enhancing accuracy, efficiency, and personalization. This blog explores the shift from manual methods to digital tools, detailing how big data, machine learning, and predictive analytics are revolutionizing patient care and accelerating research. Explore key benefits, applications, and future trends shaping the future of healthcare.

Discover the pivotal role Institutional Review Boards (IRBs) play in safeguarding ethical standards in clinical research. This blog delves into the origins, responsibilities, and challenges faced by IRBs as they oversee research involving human subjects. From ensuring informed consent and protecting vulnerable populations to adapting to evolving research methodologies, learn how IRBs uphold the integrity of clinical trials and contribute to advancements in medical science.

Blockchain, beyond its crypto roots, offers a secure, transparent solution to clinical research challenges. It enhances data security, transparency, and trust by providing an immutable, decentralized ledger. Discover its impact on safeguarding patient information, promoting open reporting of trial results, and navigating the evolving regulatory landscape. Explore real-world examples and envision the future of clinical research with blockchain technology.

Explore how real-world data (RWD) is reshaping clinical research by integrating insights from electronic health records, wearables, and patient feedback. Our blog covers RWD's benefits, challenges, and future trends, illustrated with impactful case studies. Discover how RWD is enhancing research relevance and patient care.
Clinical trial transparency, the open sharing of trial data, is vital for trust, data accuracy, and research validation. This blog examines its evolution due to data suppression cases and stricter regulations, its benefits and challenges, and its impact on various stakeholders. Understanding transparency's role is crucial for advancing medical research and improving patient outcomes.

Biostatistics is crucial in clinical research, ensuring accuracy and reliability by integrating statistical methods into study design and data analysis. This blog explores how biostatistics transforms raw data into meaningful insights, enhancing the credibility of research findings and advancing healthcare. Discover the vital role of biostatistics in developing new therapies and improving patient outcomes.

Explore how blockchain technology is revolutionizing clinical research by enhancing data security, transparency, and efficiency. This blog examines blockchain's potential to address challenges like data integrity, patient recruitment, and regulatory compliance. Discover real-world applications and future trends showcasing blockchain's promise in creating more trustworthy and efficient research processes.

The landscape of clinical research is transforming with the rise of decentralized clinical trials (DCTs), driven by advancements in digital technologies. DCTs minimize the need for in-person visits, enabling patients to participate from home, thus enhancing convenience and broadening access. This blog explores the significant benefits of DCTs, such as increased patient participation, improved data quality, and cost efficiency, while also addressing the challenges they pose, including data privacy, regulatory compliance, and technological barriers. Discover how DCTs are reshaping clinical research and the future prospects of this innovative approach.

Explore the transformative impact of big data in clinical research, from enhancing patient outcomes to driving groundbreaking discoveries. This blog delves into how vast datasets from electronic health records, genomic sequencing, and wearable devices are revolutionizing healthcare. Discover the potential of big data to streamline operations, refine trial designs, and personalize treatment plans. Understand the challenges of data integration, privacy concerns, and ethical considerations, while exploring future trends in precision medicine and real-time analytics. Join us as we uncover the future of data-driven precision health.
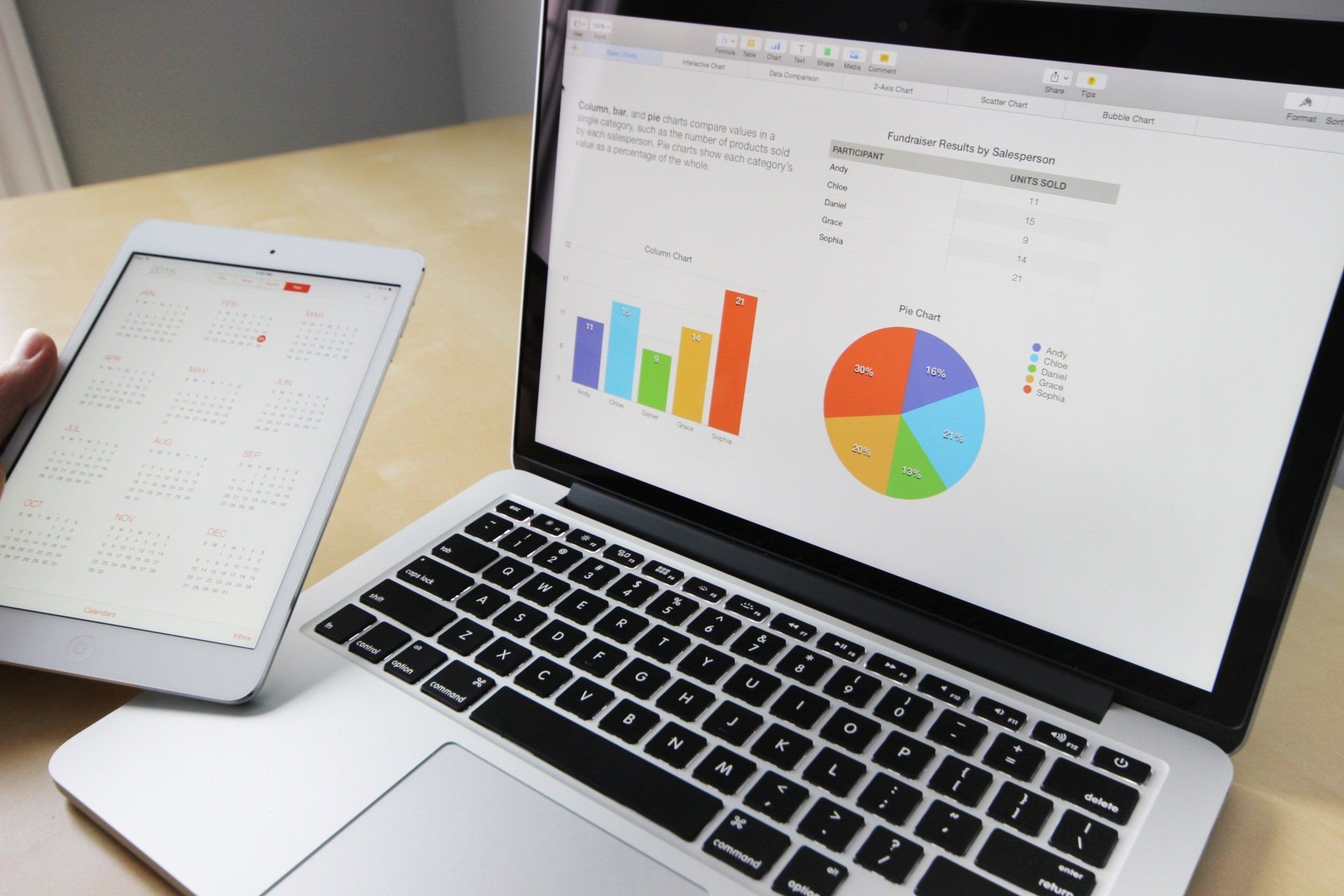
In the ever-evolving landscape of healthcare, effective patient data management is pivotal. This blog explores the latest innovations in patient data management, highlighting how cutting-edge strategies and technologies are transforming clinical research. Learn how advanced analytics, real-time data integration, and patient-centric approaches are driving significant improvements in clinical decision-making and patient outcomes. Discover the key benefits of these innovations, including enhanced data accuracy, improved patient engagement, and accelerated development of personalized treatments, ensuring the healthcare industry meets the demands of modern medicine.

In the dynamic landscape of biomedical research, regulatory frameworks play a pivotal role in ensuring the safety, efficacy, and ethical conduct of scientific investigations. As our understanding of diseases and therapeutic modalities evolves, and as new technologies emerge, regulatory agencies must continuously adapt and update their guidelines and policies to keep pace with these advancements. Regulatory updates can have far-reaching implications for researchers, sponsors, and ultimately, patient care. This blog explores the role of regulatory agencies, the impact of regulatory updates on biomedical research, and best practices for navigating these changes effectively.
The landscape of clinical research is undergoing a transformative shift with the rise of decentralized clinical trials (DCTs). These innovative trial models are revolutionizing patient engagement by offering a more convenient, accessible, and patient-centric experience. As the healthcare industry continues to embrace digital technologies, DCTs are poised to become a cornerstone of modern clinical research, addressing long-standing challenges and reshaping traditional paradigms.










